
Connection shown between glucose metabolism and colorectal tumorigenesis
An international team of researchers, led by Dr Carlos Sebastián Muñoz, a Beatriz Galindo researcher and director of the UB Metabolic Dynamics in Cancer Laboratory at the Barcelona Science Park, has identified a type of cells in the intestines, characterised by their high glucose metabolism, that behave like stem cells and could be responsible for the genesis and spread of colorectal cancer. The results of the study, published in Nature Communications, open up a new path for research to design new, more effective therapeutic targets for this type of tumour, based on blocking the metabolic reprogramming of these cells.
The study was led jointly by Dr Carlos Sebastián and Prof Raul Mostoslavsky from the Massachusetts General Hospital Cancer Center (associated with Harvard Medical School), with participation from renowned researchers at the Candiolo Cancer Institute-FPO (Turin, Italy) and several hospitals and departments of Harvard University.
Colorectal cancer is one of the most common types of cancer in Spain, in both women and men, with 40,000 cases diagnosed in 2021 according to the Asociación Española Contra el Cáncer (AECC). Despite recent advances in diagnosis and treatment of this type of cancer, its mortality rate remains very high: the second most common cause of cancer-related deaths. So, it is important to get a better understanding of the mechanisms involved in the genesis and spread of these tumours.
Role of glucose metabolism in intestinal tumours
Cancerous cells don’t metabolise glucose in the same way as normal cells. “In previous studies, we showed that glucose metabolism plays a key role in the initiation of intestinal tumours in murine models of colorectal cancer. Now, in this study, we’ve discovered a rare population of cells in these tumours characterised by their high rates of glucose metabolism (glycolytic cells) and that behave like stem cells, meaning they are pluripotent and can initiate a tumour,” explains Dr Carlos Sebastián, member of the Department of Cell Biology, Physiology and Immunology in the Faculty of Biology at the University of Barcelona (UB) and the Institute of Biomedicine of the University of Barcelona (IBUB).
The results show that intestinal tumours are metabolically heterogeneous and suggest that there is a hierarchy in which a small population of highly glycolytic cells are responsible for tumour initiation and spread.
“To analyse this heterogeneity, we developed a novel system that, in combination with intestinal organoids (3D cultures that imitate the cell composition of the intestine), has allowed us to visualise, track and characterise cells with different metabolic properties in the intestinal epithelium and in tumours at the single-cell level. The characterisation of these glycolytic cells has led us to discover that they act as intestinal stem cells and that this cell type is also found in intestinal tumours,” explains Dr Sebastián.
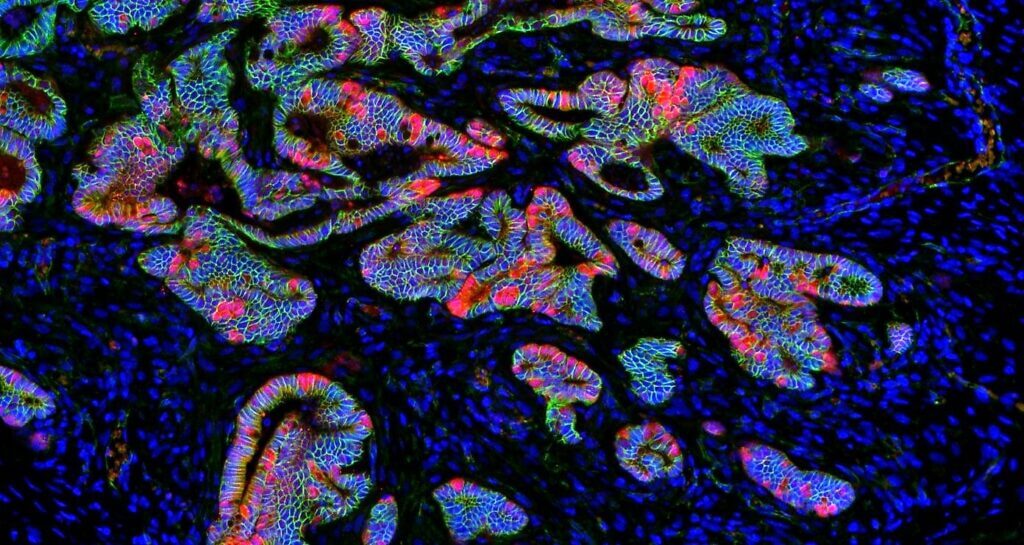
Immunofluorescence image showing cells with different metabolic properties within the intestinal epithelium (Author: Carlos Sebastián).
Metabolic reprogramming in the tumour process
One of the main characteristics of tumour cells is their ability to change their metabolism (metabolic reprogramming) to grow, survive and proliferate, which is intimately linked to tumour progression, metastasis and therapeutic resistance.
Traditionally, the scientific community has considered these metabolic alterations mere adaptations geared towards providing the energy and macromolecules needed for cell proliferation. “However, our studies show that this metabolic reprogramming occurs at very early stages during tumorigenesis and is intrinsically associated to cell fate determination,” reveals the scientist.
Plus, the team of researchers has discovered that blocking metabolic reprogramming is enough to inhibit the glycolytic cells’ ability to form intestinal tumours. “Given that these tumour-initiating cells are involved in tumour progression, metastasis and relapse, our results suggest that this metabolic vulnerability could be used to treat colorectal cancer,” he adds.
Another important conclusion from the study is that these glycolytic cells are quiescent, meaning they don’t proliferate, which challenge the dogma that this metabolic reprogramming is associated with biomass production and cell proliferation. In fact, the researchers observed that these metabolic characteristics are necessary to protect these cells from oxidative stress and maintain their stem cell potential.
“Although these studies were conducted with mouse models of colon cancer and, therefore, we must be prudent in transferring them to the human disease, the results obtained could be useful in the future for designing new, more effective therapies for this type of cancer. In this regard, our laboratory continues working in this line with samples from colon cancer patients to apply our findings to these patients,” the researcher highlights.
» Reference article: Sebastián C*, Ferrer C, Serra M, Choi JE, Ducano N, Mira A, Shah MS, Stopka SA, Perciaccante AJ, Isella C, Moya-Rull D, Vara-Messler M, Giordano S, Maldi E, Desay N, Capen DE, Medico E, Cetinbas M, Sadreyev RI, Brown D, Rivera MN, Sapino A, Breault DT, Agar NYR, Mostoslavsky R*. “ A non-dividing cell population with high pyruvate dehydrogenase kinase activity regulates metabolic heterogeneity and tumorigenesis in the intestine”. Nature Communications (2022). DOI: https://doi.org/10.1038/s41467-022-29085-y




