IBEC discovers a key role of protons and superoxide ions in the respiratory chain
A new study led by the Institute for Bioengineering of Catalonia (IBEC), located at the Barcelona Science Park, in collaboration with the University of Barcelona (UB) and the Institute for Chemical Research – cicCartuja of the Spanish National Research Council (CSIC) and the University of Seville, reveals that protons and reactive oxygen species act as mediators in long-distance charge transport within the mitochondrial respiratory chain, a fundamental process in cellular respiration. The conclusions of this study, published in the journal Small, are key, since mitochondria act as the powerhouses of all cells and their alteration is associated with numerous diseases.
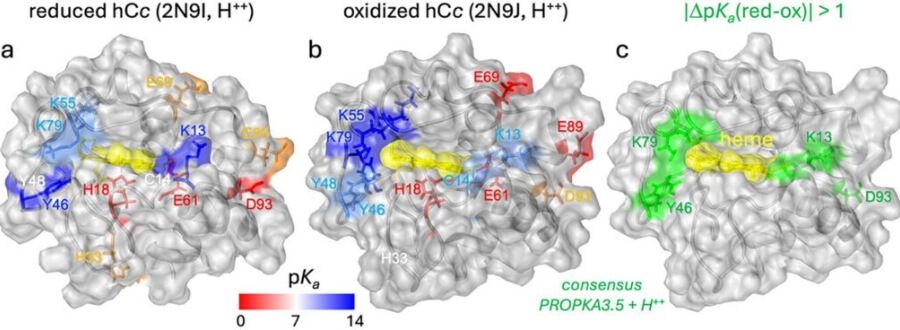
The researchers, led by Pau Gorostiza, ICREA research professor and head of the Nanoprobes and Nanoswitches group at IBEC, together with Anna Lagunas, senior researcher in the Nanobioengineering group at the same center, have discovered that long-distance charge transport between two key proteins of the mitochondrial respiratory chain — cytochrome c and respiratory complex III — is mediated by protons and the superoxide ion, a reactive oxygen species.
Despite its fundamental character, this work has twofold impact: it helps to improve our understanding of the regulation of cellular respiration and could inspire new applications in emerging fields. “Understanding these basic mechanisms is essential because mitochondria are the powerhouses of cells, and their dysfunction is linked to many diseases”, Lagunas explains, first author. “Furthermore, this discovery could inspire the development of new protonic devices, equivalent to electronic devices but operating with a positive charge”, adds Gorostiza.
This work culminates a line of research that these teams have been developing collaboratively for years. The first results of this research were published in 2018, when the researchers demonstrated that two proteins could transfer electrons over long distances through an aqueous solution without forming a stable complex. Subsequently, in 2022, a second study was published, revealing how phosphorylation regulates this process and its relevance in cell signalling. The new article completes this ‘trilogy’, as Pau Gorostiza puts it, “they are three pieces of the same story. This chapter leaves some intriguing questions unanswered, but gives us a much broader perspective on how this fundamental mechanism may work”.
Although the transport of electrons within a single protein or complex has been studied in detail using structural and functional techniques, the transfer of electrons between proteins remains somewhat mysterious. This is due to the presence of the aqueous solvent, as well as the dynamic and transient nature of the molecular interaction between proteins and the electron transfer event itself. The new work by IBEC addresses this challenge by using nanometric and single-protein techniques to allow observation of a fundamental process that, until now, had been very difficult to investigate using macroscopic techniques.
The fundamental role of protons
To discover the ‘mediator’ of long-distance electronic transport through water, the team carried out several experiments. First, they varied the acidity of the solution (i.e. the concentration of protons) within ranges compatible with protein stability, observing that transport was more efficient under slightly acidic conditions (with a higher concentration of protons). Next, they replaced the usual medium with heavy water — a variant of water in which the hydrogen atoms are replaced by deuterium, a heavier form of hydrogen — and found that this hindered the process. These results both point to the essential role of protons in charge transport. Finally, they repeated the measurements in solutions with different concentrations of dissolved oxygen and found that the absence of oxygen reduced the distance over which proteins could transport charges.
As Lagunas explains, “these results indicate that protons and oxygen play a central role in this mechanism. Everything points to a proton-coupled electron transfer (PCET) process, whereby the exchange of an electron is closely linked to that of a proton. This process could involve proton transport mechanisms such as Grotthuss, whereby chains of water molecules pass the proton to each other as if holding hands”.
The researchers also suggest that the superoxide anion, a relatively stable reactive oxygen species that is naturally produced by complex III, could also act as a mediator in this process.
From a biological perspective, Professors Irene Díaz-Moreno and Miguel A. de la Rosa emphasise the importance of this discovery. They explain that “the efficiency of mitochondrial respiration directly affects a cell’s ability to produce ATP, the ‘energy currency’ that sustains all vital processes. In a crowded cellular environment, optimising electron transfer is essential in order to make the most of the available energy while avoiding losses and reducing the uncontrolled production of reactive oxygen species. Therefore, understanding how protons and superoxide mediate long-distance transfer expands our basic knowledge and provides insight into how cellular energy efficiency is regulated, as well as what happens when this process is disrupted in metabolic and degenerative diseases”.
» Article of reference: Anna Lagunas, Alexandre M. J. Gomila, Alba Nin-Hill, Alejandra Guerra-Castellano, Gonzalo Pérez-Mejías, Josep Samitier, Carme Rovira, Miguel A. De la Rosa, Irene Díaz-Moreno, Pau Gorostiza. Long-Distance Charge Transport between Cytochrome c and Complex III is Mediated by Protons and Reactive Oxygen Species. Small (2025). doi: 10.1002/smll.202501286
» Link to the news: IBEC website [+]




